Two products were approved for listing in one month, and Forerunner Medical transportation platform was quickly built

On July 7, 2021, Forerunner Medical device technology (Shanghai) Co., Ltd. announced that its self-developed orthopaedic implant subdivision product "non absorbable ligament fixing screw" was registered and approved by the State Drug Administration (nmpa) for listing, which is suitable for ligament repair and reconstruction.
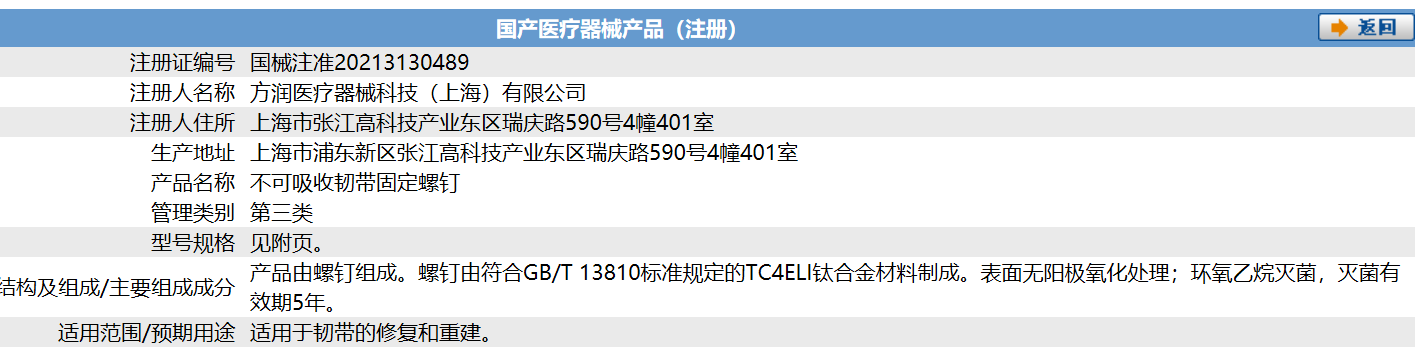
This is the second product approved by Forerunner Medical in the field of orthopedic devices and implants after the "non absorbable thread anchor" product in the field of sports medicine was approved to market in late June. This means that in orthopedic clinic, Forerunner Medical not only has the plasma system with high technical barriers necessary for surgery to assist doctors in minimally invasive surgery, but also has a series of implant products with high-value consumables to help patients get a better and more economical treatment experience in the repair, fixation and reconstruction of tendons and ligaments.
Taking the plasma system with high technical barriers as the starting point, build a complete surgical solution for sports medicine
With the improvement of living standards, sports in China continue to be popularized, sports groups continue to expand, and the probability of related orthopedic injuries also increases rapidly. Taking the marathon and long-distance running advocated by modern urban people as an example, it can potentially cause nearly 15 kinds of sports injuries, and even cause great damage to the knee. Because sports injury diseases are more common in young people, for example, in ball games, young people are easy to cause ligament rupture due to excessive force and fierce collision. In order to reduce treatment trauma and quickly restore sports ability, most young groups receive ligament reconstruction surgery under arthroscopy, which plays a great role in promoting the development of new methods of minimally invasive treatment and the application of new materials.
The key to the treatment of ligament injury lies in the repair of the damaged ligament. Some tears can be repaired by direct suture, and complete rupture requires surgery to transfer, repair and reconstruct the adjacent tendon, fascia and other tissues. In this process, the screws used to fix the ligament are very important. They are used to fix the broken part of the ligament or connect the ligament with the torn bone. Absorbable nails are commonly used in ligament repair surgery, which are mainly used for the reconstruction of middle broken cruciate ligament; Non absorbable metal screws are used for the repair operation with bone blocks at the starting and ending points. If the lateral collateral ligament is broken, it may be fixed with metal rivets.
Forerunner Medical has been committed to building a complete surgical solution for sports medicine since the establishment of the company, and takes the plasma system with high technical barriers as the entry point into the field of sports medicine. It is reported that the approved products are only one in the field of sports medicine pipeline with complete layout, and many products will be approved and listed in the future.
At present, based on Forerunner Medical's existing two core technology platforms of plasma ablation technology and RF ablation, the company has formed a complete technical solution and sales platform in the fields of sports medicine, spine, ear, nose and throat, tumor intervention and so on, Its diversified independent product lines built around clinical needs have the strength to compete with international first-line medical device R & D companies.
According to Wang Yu, the person in charge of Forerunner Medical operation, in the follow-up, Forerunner Medical will also have multiple implant product registration certificates approved. Implants are an indispensable and important product series in the whole sports medicine surgical solution. Forerunner Medical will form a pattern of taking the isoionic system mastering the core technology as the fist product to drive the sales of implant series products in the future.
It is reported that under the guidance of the national centralized procurement policy, as a technology platform enterprise focusing on innovation and R & D, Forerunner Medical also actively strives for, on the one hand, strengthening the R & D declaration of its own innovative products, on the other hand, expanding the production platform and space, cooperating with mass production, and reducing R & D and production costs; Build the products into the mainstream of domestic substitution for imported products to benefit the majority of patients, so as to form a pattern of positive competition between domestic innovative medical device enterprises and traditional international multinational corporations in the field of transportation and medicine.
Mr. Qin Jay, founder / Chairman of Forerunner Medical, said:
"With the support of China's policy to encourage the development of local innovative medical device enterprises, Fang run medical has entered a period of rapid development. We will diversify products around the" clinical pain spot "in the field of sports medicine, strengthen our independent research and development, meet more clinical needs, and provide the best surgical instruments for the vast number of Chinese doctors. Provide patients with safe and effective "made in China" medical devices. We hope that Forerunner Medical will become a platform enterprise with the strongest active device technology and the most complete overall solution in the field of sports medicine in China. "
About Forerunner Medical group
Founded in 2009, Forerunner Medical group is headquartered in the medical device industry base of Zhangjiang Science City, Shanghai. It is a platform enterprise committed to the R & D and production of innovative minimally invasive medical devices; The company has three high barrier core technology platforms: plasma ablation technology, radiofrequency ablation and implants, and its business covers four fields: sports medicine, spinal minimally invasive surgery, ear, nose and throat, and tumor intervention. The company has established a talent team with rich experience in product design, quality control, clinical verification and market development. At present, it has obtained 14 nmpa registrations, 65 national patents, CE product certification and ISO 13485 quality system certification. The core product technology has been applied to more than 1000 hospitals in China, and has reached strategic cooperation with international medical device company Terumo in Europe. The core technology products are sold in Europe, Southeast Asia, Australia and other places.




