Forerunner Medical implant products have been approved by the State Food and drug administration, creating the first domestic enterprise with a complete product line platform
((Shanghai, China) on June 23, 2021, the official website of China National Drug Administration (nmpa) showed that the "non absorbable tape anchor" of Forerunner Medical device technology (Shanghai) Co., Ltd. had been approved in China, This means that Forerunner Medical, which "takes clinical demand as the guidance and adheres to independent innovation as the development strategy", has officially become the first company in China to have a complete product line layout in the field of Sports Medicine - a series of implant products with both high technical barriers necessary for surgery and high value and material consumption, It has formed a pattern of positive competition between domestic innovative medical device enterprises and traditional international multinational corporations in the field of medical transportation.
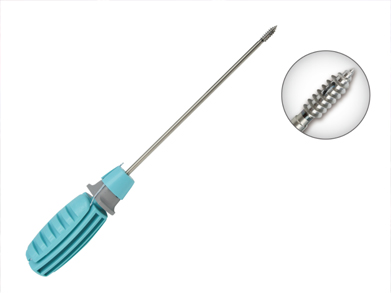
Non absorbable suture anchor belongs to the field of sports medicine, which is composed of anchor, suture and inserter. It is suitable for the repair, fixation and reconstruction of tendon and ligament, and the connection and fixation of bone and soft tissue.
Forerunner Medical has been committed to building a complete surgical solution for sports medicine since its inception, and takes the plasma system with high technical barriers as the entry point into the field of transportation medicine. At present, the company's products have obtained 14 nmpa registrations, 65 national patents, including 9 invention patents, CE product certification and ISO13485 quality system certification. Based on the company's existing two core technology platforms of plasma ablation technology and RF ablation, Forerunner Medical has formed a complete technical solution and sales platform in the fields of sports medicine, spine, ear, nose and throat, tumor intervention and so on, Its diversified independent product lines built around clinical needs have the strength to compete with international first-line medical device R & D companies.
It is reported that the follow-up company will also have a number of implant product registration certificates approved. As an indispensable and important product series in the whole sports medicine surgical solution, Forerunner Medical will form a pattern of taking the plasma system mastering the core technology as the fist product to drive the sales of implant series products in the future.
With the economic development and the improvement of national living standards, the market demand for clinical diagnosis and treatment in the field of sports medicine is gradually upgrading. One kind of treatment population is the aging population. With the increase of age, degenerative lesions of joint soft tissue will occur, and sports injury will also occur in the process of normal life; The other is sports people. As more and more people pay attention to health and maintain long-term exercise, injuries during exercise, such as knee ligament injury and meniscus tear, have also become high-risk diseases. There is an urgent need for better clinical solutions and "high-quality and low-cost" domestic substitutes more suitable for Chinese patients.
In order to meet the market demand, Forerunner Medical has vigorously accelerated the expansion and approval of the new plant after completing the round B financing last year. It is planned that the new plant of the company will be submitted for approval and put into operation next year, and the production capacity will be doubled at that time.
In response to the approval of this product, Wang Yu, head of Forerunner Medical operation, said:"In the future, Forerunner Medical Association will strengthen innovation and exploration in the whole line of product development in the field of sports medicine. With the rapid spread of medical operation in the country, we will continue to independently develop innovative products around the clinical pain points and the special clinical operation needs of Chinese doctors, so as to provide the best surgical instruments for the majority of Chinese doctors and provide safe and effective medical services for the majority of patients "Made in China" medical devices
Mr. Qin Jay, founder / Chairman of Forerunner Medical, said:
"In the future, we will continue to devotes ourselves to the development of independent innovation products. With the support of the policy of encouraging the development of local innovative medical device enterprises, Fang run healthcare has entered a period of rapid development. We hope that with the accumulation of experience gained in the past more than 10 years and platform construction, we can diversify products in the field of sports medicine and satisfy more clinical types. Demand, our short-term goal is to become a platform enterprise with the strongest active device technology and the most complete overall solution in the field of sports medicine in China. "
About Forerunner Medical group
Founded in 2009, Forerunner Medical group is headquartered in the medical device base in the East District of Zhangjiang Science City, Shanghai. It is a platform enterprise committed to the R & D and production of innovative minimally invasive medical devices; The company has three high barrier core technology platforms: plasma ablation technology, radiofrequency ablation and implants, and its business covers four fields: sports medicine, spinal minimally invasive surgery, ear, nose and throat, and tumor intervention. The company has established a talent team with rich experience in product design, quality control, clinical verification and market development. At present, it has obtained 14 nmpa registrations, 65 national patents, CE product certification and ISO 13485 quality system certification. The core product technology has been applied to more than 1000 hospitals in China, and has reached strategic cooperation with international medical device company Terumo in Europe. The core technology products are sold in Europe, Southeast Asia, Australia and other places.




